Pipeline
MonoTx™, cord blood MNCs are used to treat people with chronic spinal cord injury as well as research material for pre-clinical (animal) studies to develop therapies for hypoxia-ischemia encephalopathy, acute and chronic stroke, and age-related macular degeneration.
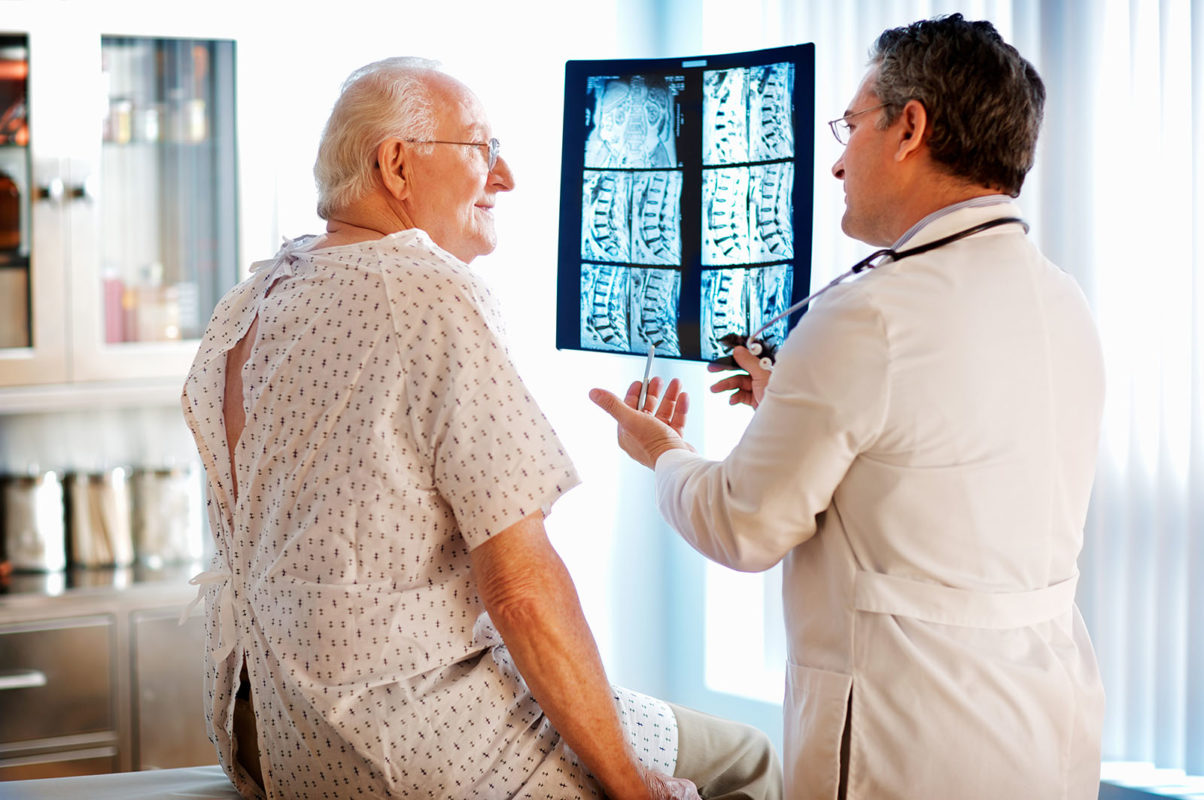
Spinal Cord Injury (SCI)
Phase I–II Clinical Trial Assessing the Safety and Efficacy of Umbilical Cord Blood Mononuclear Cell Transplant Therapy for Chronic Complete Spinal Cord Injury.
About 3 million people worldwide have chronic SCI. No treatment exists that restores function with those having chronic complete spinal cord injury. In phase I and II clinical trials, Prof Young showed that transplantation of umbilical cord blood mononuclear cells (UCBMNC) injected into the spinal cord above and below the injury site will promote growth of axons across the injury. If the patients then undergo intensive rehabilitation – walking 6 hours a day, 6 days a week, for 6 months, as many as 75% of the patients recover long distance ambulation, bowel and bladder function within one year following transplantation. MonoTx™ is planning a clinical trial in the US (New York, New Jersey, and Pennsylvania). If this phase II trial successfully restores function in the majority of people with chronic complete spinal cord, we will proceed to phase III trials with the intention of obtaining regulatory approval in the US, Europe, China, India and South America.
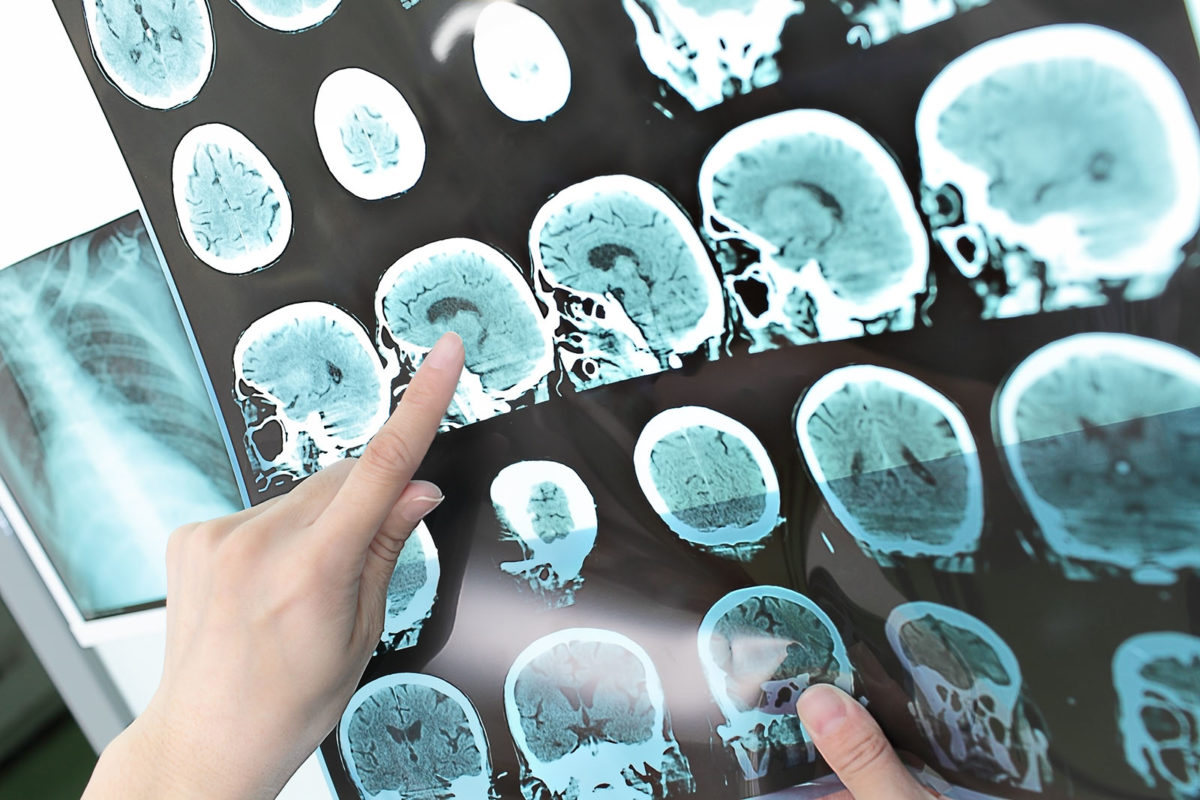
Hypoxic-Ischemia Encephalopathy (HIE)
Phase I trial Assessing Feasibility and Safety of Umbilical Cord Blood Transfusion in the Treatment of Neonatal Cerebral Ischemia and Anemia.
About 1 out of every 200 newborn babies suffer from HIE annually. HIE results when babies are in the birth canal for prolonged periods, have reduced blood flow, hypoxia, or anemia. This trial is being conducted in collaboration with the Chinese University of Hong Kong. Pre-clinical studies suggest that hypoxia induced (could use alternative for hypoxia inducted) 7-day old rats administered UCB MNC intravenously show significant behavioral improvement, reduced neuronal loss, and survival. The human trial will utilize the baby’s autologous blood cells infused intravenously. Cord blood will be collected from the cord and placenta at the time of birth and kept for 24 hours in the hospital. If hypoxia ischemia occurs at birth, the whole cord blood will be given during the first 24 hours. If hypoxia ischemia occurs later than 24 hours, mononuclear derived cord blood cells processed at MonoTx™ will be infused.
Acute Cerebral Stroke
Stroke is the leading cause of death and disability in adults. Most strokes result from clots (emboli) or other causes of occlusion that block blood vessels in the brain, or intracerebral hemorrhage caused by rupture of small arteries. About 80 million people, worldwide, have chronic stroke and about 8 million people suffer a stroke every year. Most people improve after their first stroke within a year if they receive intensive rehabilitation but many continue to have residual neurological deficits. We will carry out two clinical trials, one to assess the effect of the cord blood on acute stroke and the other on chronic stroke. All the subjects will receive intensive rehabilitation and locomotor training after treatment. The dose given will depend on the location and size of the brain infarct.

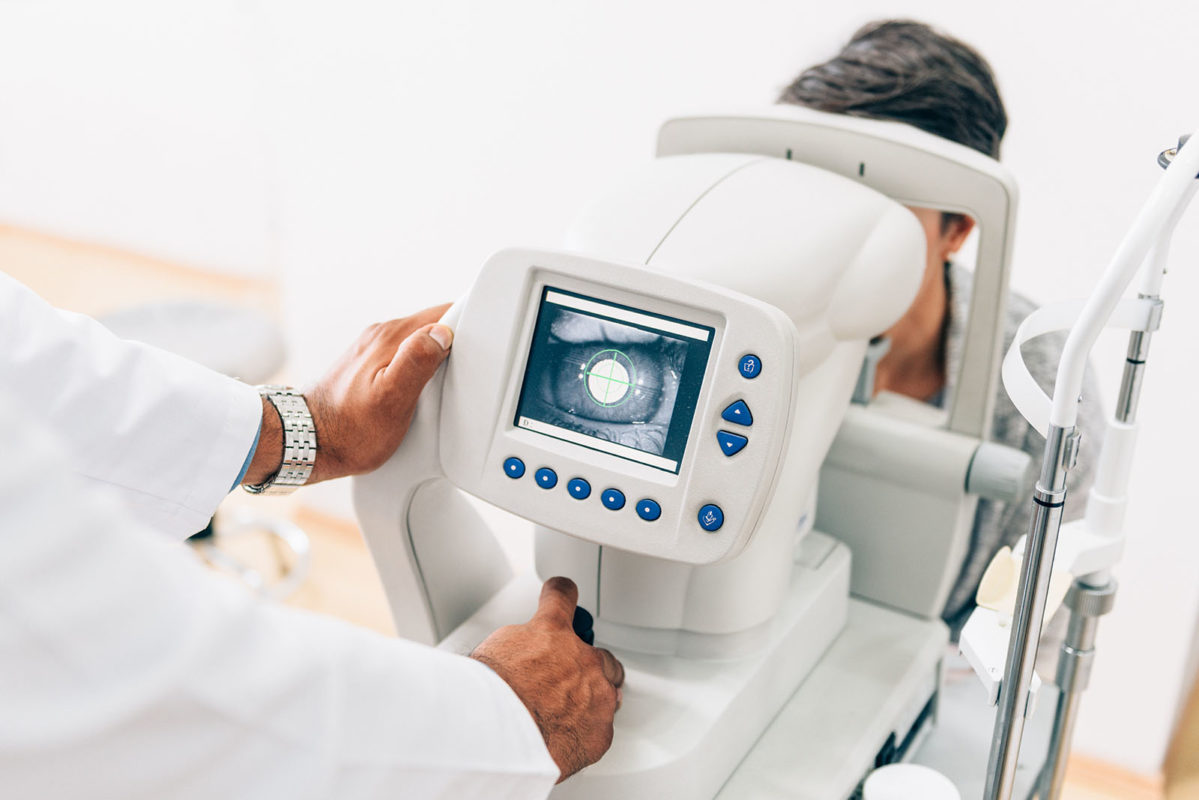
Age-related Macular Degeneration (AMD)
Age-related macular degeneration (AMD) is an eye disease that may get worse over time. It’s the leading cause of severe, permanent vision loss in people over age 60.
About 200 million people currently suffer from AMD, which causes degeneration of the retina and neural connection between the retina and the brain. MonoTx is currently studying the effects of a MNC treatment on AMD, following injection of mononuclear cells into the subretinal space. The goal of MNC therapy on AMD differs significantly from other AMD therapies. Instead of preventing AMD, our goal is to replace the ganglionic neurons that send axons into the optic nerve. Thus, the goal is not to regenerate the damaged ganglionic neurons but to stimulate their replacement.


 中文 (香港)
中文 (香港)